Comparison of Insulins (United States)
Click HERE to access the Canadian version of this resource.
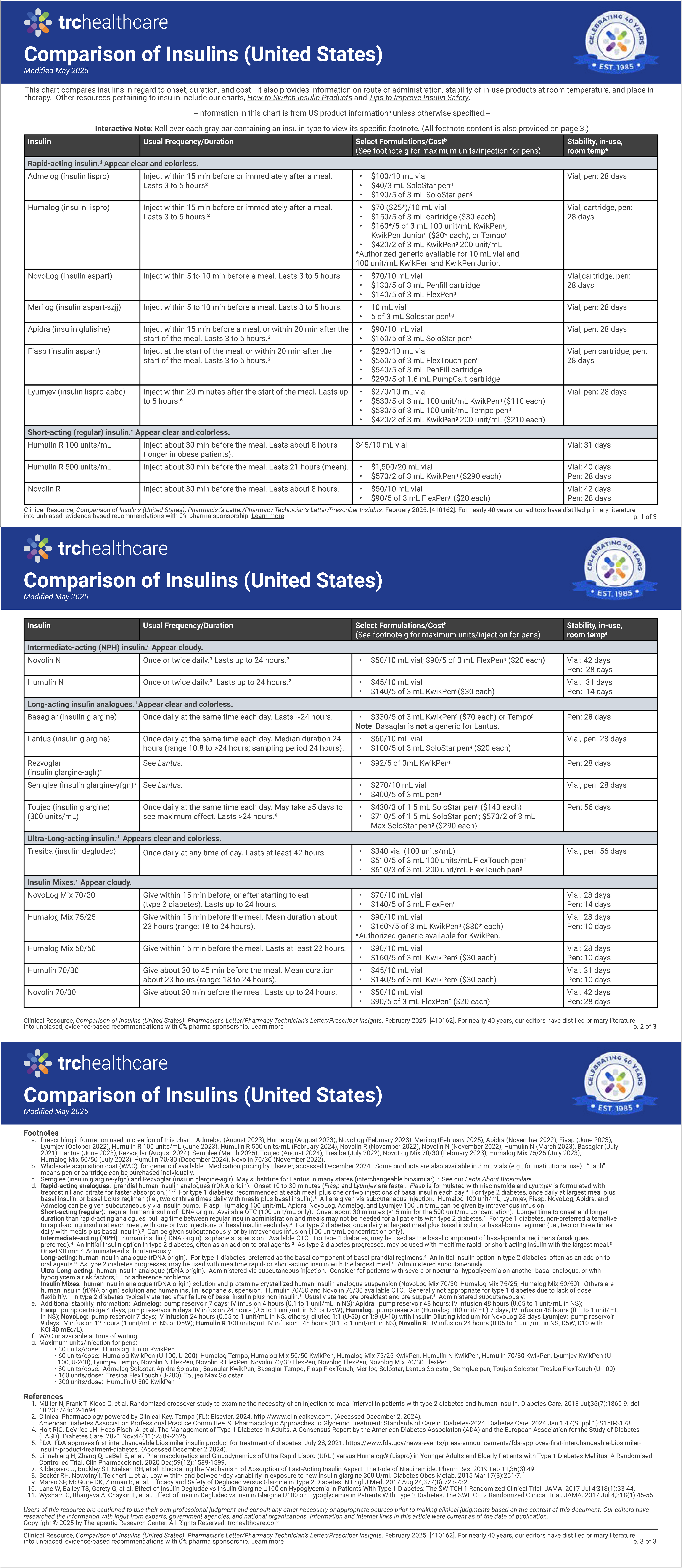
This chart compares insulins in regard to onset, duration, and cost. It also provides information on route of administration, stability of in-use products at room temperature, and place in therapy. Other resources pertaining to insulin include our charts, How to Switch Insulin Products and Tips to Improve Insulin Safety.
--Information in this chart is from US product information a unless otherwise specified.--